- Home
- boston scientific spinal cord stimulator surgery
- Boston's WaveWriter SCS systems gain expanded non-surgical back pain indication from US FDA
Boston's WaveWriter SCS systems gain expanded non-surgical back pain indication from US FDA
5 (773) · $ 11.99 · In stock
Boston Scientific Corporation announced today that the US Food and Drug Administration (FDA) has approved an expanded indication of the company’s WaveWriter spinal cord stimulation (SCS) systems for the treatment of chronic low back and leg pain in people with non-surgical back pain (NSBP). ″Diagnosing and treating chronic low back pain can be challenging,” said James […]

NeuroNews on LinkedIn: Galaxy SEAL device demonstrates positive one-year outcomes

The FDA has approved an expanded indication of the WaveWriter SCS

Boston Scientific Pain Management on LinkedIn: #nans24

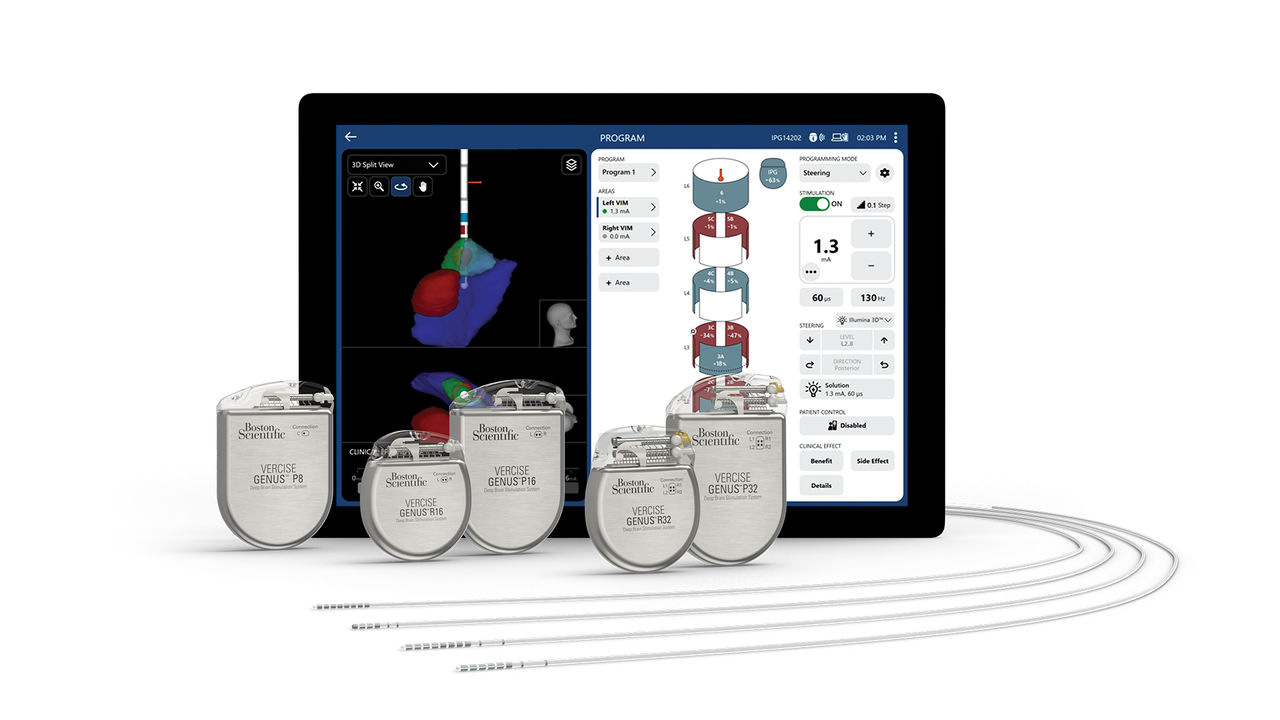
Boston Scientific launches Vercise primary cell and Vercise Gevia deep brain stimulation systems

NeuroNews on LinkedIn: WSO predicts 50% more deaths unless “catastrophic” gaps in global stroke…

Spinal Cord Stimulation (SCS) For Chronic Pain
Boston's WaveWriter SCS systems gain expanded non-surgical back pain indication from US FDA

Boston Scientific announces agreement to acquire Devoro Medical

Expanded Indications for SCS - Boston Scientific

Nevro announce launch of a new medical device for the treatment of chronic pain

INS 2022: Dorsal horn dendrite stimulation offers “true breakthrough” in pain management field - NeuroNews International

Spinal cord stimulation